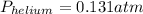Answer:
Hi, the posted question has some missing part. In the given question, there is no information about presence of helium gas inside cylinder. But, some amount of helium gas should be present inside cylinder.
Pressure of helium is 0.131 atm
Step-by-step explanation:
For a mixture of ideal gases, we can apply dalton's law of partial pressure to determine partial pressure of a particular gas by using the following equation:
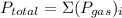 , where
, where
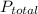 is total pressure of mixture and
is total pressure of mixture and
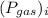 is the partial pressure of 'i'th gas.
is the partial pressure of 'i'th gas.
Here
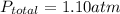
Let's assume all three gases (nitrogen, oxygen and helium) behaves ideally.
So,
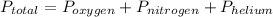
So,
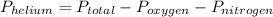
So,
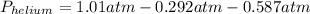
So,