Answer:
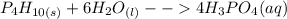
Step-by-step explanation:
To produce the balanced chemical equation, it is important to know the chemical formular of the individual compounds.
Solid tetraphorous decaoxide,
From the name, we can tell that it contains four (tetra) phosphorus (K) atoms and ten (deca) oxygen atoms so its formular is written as
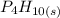 The formular has a subscript of s because it is in its solid state.
The formular has a subscript of s because it is in its solid state.
liquid water,
The chemical formula of water is given as;
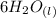 It has a subscript of l because it is in its liquid state.
It has a subscript of l because it is in its liquid state.
Aqueous Phosphoric acid,
The chemical formula of Phosphoric acid is given as;
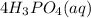 It has a subscript of aq because it is in its aqueous state. The formular matches the hint that stated it contains three hydrogen atoms.
It has a subscript of aq because it is in its aqueous state. The formular matches the hint that stated it contains three hydrogen atoms.
Now we write out the equation with what we know so far;
Solid tetraphosphorus decaoxide + liquid water --> aqueous phosphoric acid
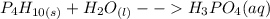
Balancing the equations by adding numbers that makes the total number of atoms on the reactant side equal to that on the product side leads to;
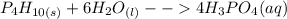
Ignore the A symbol thatkeeps showing up after the water H2O, i can't seem to be able to remove it.