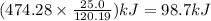Answer:
Amount of heat released when 25.0 g of
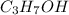 (rubbing alcohol) is combusted equal to 98.7 kJ
(rubbing alcohol) is combusted equal to 98.7 kJ
Step-by-step explanation:
According to balanced equation (as given in problem), combustion of 2 moles of rubbing alcohol release 474.28 kJ of heat.
Molar mass of
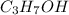 (rubbing alcohol) = 60.095 g/mol
(rubbing alcohol) = 60.095 g/mol
We know, number of moles of a compound is the ratio of mass to molar mass of that compound.
So, 2 moles of
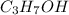 (rubbing alcohol) =
(rubbing alcohol) =
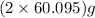
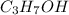 (rubbing alcohol) = 120.19 g of
(rubbing alcohol) = 120.19 g of
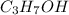 (rubbing alcohol)
(rubbing alcohol)
So, combustion of 120.19 g of rubbing alcohol release 474.28 kJ of heat.
Hence amount of heat released when 25.0 g of
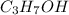 (rubbing alcohol) is combusted =
(rubbing alcohol) is combusted =