Answer:
![[SO_2Cl_2]_(600)= 0.0842 M](https://img.qammunity.org/2020/formulas/chemistry/college/2xbrey0y6yc01zoiu2pfi4cblzx88wsr7a.png)
Step-by-step explanation:
Some theoretical knowledge is required here. We should understand that whenever we plot the natural logarithm, ln, of a concentration vs. time and obtain a straight line, this indicates a first-order reaction. That said, since this is the case here, we have a first-order reaction with respect to
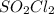 .
.
The linear equation has the following terms:
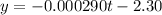
It is a linear form of the integrated first-order law equation:
![ln[SO_2Cl_2]_t = -kt + ln[SO_2Cl_2]_o](https://img.qammunity.org/2020/formulas/chemistry/college/ws0h4wv2jt4paeqn26j3u4h47farmo3jhb.png)
Therefore, the rate constant, k, is:
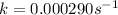
The natural logarithm of initial molarity is:
![ln[SO_2Cl_2]_o = -2.30](https://img.qammunity.org/2020/formulas/chemistry/college/wc9ej2nfnb36cvrsnwdxndng72oit7sxwp.png)
Using the equation, we may substitute for t = 600 s and obtain the natural logarithm of the concentration at that time:
![ln[SO_2Cl_2]_(600) = -0.000290 s^(-1)\cdot 600 s - 2.30 = -2.474](https://img.qammunity.org/2020/formulas/chemistry/college/137pn438vf9p7pxcl3fqqhrv3txvzkpuwp.png)
Take the antilog of both sides to find the actual molarity:
![[SO_2Cl_2]_(600)=e^(-2.474) = 0.0842 M](https://img.qammunity.org/2020/formulas/chemistry/college/70koi4iub3nv2r1m643tkuzebsphwyuyy3.png)