Answer:
34.28 L ( 1.5*22.4 L)
Step-by-step explanation:
Calculation of the moles of aluminum as:-
Mass = 55 g
Molar mass of aluminum = 26.981539 g/mol
The formula for the calculation of moles is shown below:

Thus,
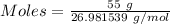
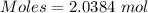
According to the reaction:-
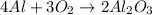
4 moles of aluminum react with 3 moles of oxygen gas
1 mole of aluminum react with
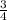 moles of oxygen gas
moles of oxygen gas
2.0384 moles of aluminum react with
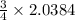 moles of oxygen gas
moles of oxygen gas
Moles of oxygen gas = 1.5288 moles
At STP,
Pressure = 1 atm
Temperature = 273.15 K
Using ideal gas equation as:

where,
P is the pressure
V is the volume
n is the number of moles
T is the temperature
R is Gas constant having value = 0.0821 L.atm/K.mol
Applying the equation as:
1 atm × V = 1.5288 mol × 0.0821 L.atm/K.mol × 273.15 K
⇒V = 34.28 L ( 1.5*22.4 L)