One of the concepts to be used to solve this problem is that of thermal efficiency, that is, that coefficient or dimensionless ratio calculated as the ratio of the energy produced and the energy supplied to the machine.
From the temperature the value is given as
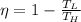
Where,
T_L = Cold focus temperature
T_H = Hot spot temperature
Our values are given as,
T_L = 20\° C = (20+273) K = 293 K
T_H = 440\° C = (440+273) K = 713 K
Replacing we have,
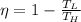
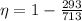
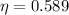
Therefore the maximum possible efficiency the car can have is 58.9%