To solve this problem it is necessary to apply the concepts related to the Kinetic Energy and the Energy Produced by the heat loss. In mathematical terms kinetic energy can be described as:
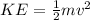
Where,
m = Mass
v = Velocity
Replacing we have that the Total Kinetic Energy is
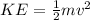
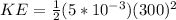
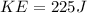
On the other hand the required Energy to heat up t melting point is
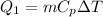
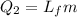
Where,
m = Mass
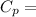 Specific Heat
Specific Heat
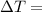 Change at temperature
Change at temperature
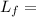 Latent heat of fussion
Latent heat of fussion
Heat required to heat up to melting point,
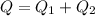
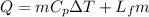
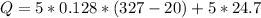
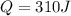
The energy required to melt is larger than the kinetic energy. Therefore the heat of fusion of lead would be 327 ° C: The melting point of lead.