Answer: Option (C) is the correct answer.
Step-by-step explanation:
It is given that partition coefficient between dichloromethane and water is 2.5. Let us assume that "x" grams of caffeine is present in 100 ml.
Hence, find the value of x as follows.
2.5 =
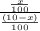
x = 25 - 2.5x
x = 7.14
or, x = 7.1
Therefore, we can conclude that caffeine extracted is 7.1 grams.