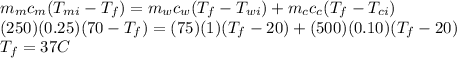Answer:
d. 37 °C
Step-by-step explanation:
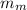 = mass of lump of metal = 250 g
= mass of lump of metal = 250 g
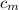 = specific heat of lump of metal = 0.25 cal/g°C
= specific heat of lump of metal = 0.25 cal/g°C
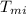 = Initial temperature of lump of metal = 70 °C
= Initial temperature of lump of metal = 70 °C
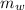 = mass of water = 75 g
= mass of water = 75 g
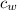 = specific heat of water = 1 cal/g°C
= specific heat of water = 1 cal/g°C
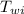 = Initial temperature of water = 20 °C
= Initial temperature of water = 20 °C
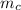 = mass of calorimeter = 500 g
= mass of calorimeter = 500 g
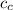 = specific heat of calorimeter = 0.10 cal/g°C
= specific heat of calorimeter = 0.10 cal/g°C
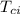 = Initial temperature of calorimeter = 20 °C
= Initial temperature of calorimeter = 20 °C
 = Final equilibrium temperature
= Final equilibrium temperature
Using conservation of heat
Heat lost by lump of metal = heat gained by water + heat gained by calorimeter