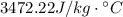Using the expression to find the Heat exchange energy, we can find the required value for the Poly's specific heat in its respectively units. Matematically this can be expressed as,
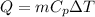
Where,
m = Mass
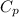 = Specific heat
= Specific heat
 = Change in temperature
= Change in temperature
Q = Heat change
Re-arrange to find the specific heat we have that
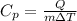
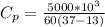
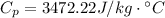
Therefore the Polly's specific heat is