Answer:
10 gm
Step-by-step explanation:
Given:
Cobalt-60 has a half life of 5.25 years.
Initial mass of the Cobalt = 40 grams
How much decayed after 10.5 years?
Solution:
The general equation for half life is given below.
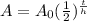 ----------(1)
----------(1)
Where A = mass of the substance that remains undecayed.
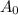 = the initial mass of the substance.
= the initial mass of the substance.
t = time
h = half life
Now, we substitute all known value in equation 1.
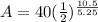
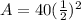
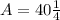
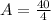
A = 10 gm
Therefore, the remaining cobalt-60 after 10.5 years is 10 gm.