Answer:
The correctly matched pair is :
 is Polar
is Polar
Step-by-step explanation:
Polarity is decided by net dipole moment . If a molecule has 0 dipole moment then it is non-polar .
If a molecule has some value of dipole moment then it is polar.Unit of dipole moment is Debye(D)
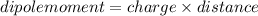
In chemical value of dipole is based on two factors :
1. There should be heteroatomic bond in a given compound..e.g H-Cl , C-N
(not C-C, N-N)
2. The molecule should be unsymmetrical in shape.
note : even if all bonds are heteroatomic but shape is symmetrical , then also dipole moment can be zero
CORRECT MATCHES
 : Polar
: Polar
It has net dipole moment of 1.85 D because its shape is bent (unsymmetrical) and all bonds are heteroatomic.
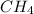 :Non-Polar
:Non-Polar
All bonds are heteroatomic(C-H) but shape is symmetrical(tetrahedral), so net dipole is zero
 : Non -Polar
: Non -Polar
All bonds are heteroatomic(C=O) but shape is symmetrical(linear), so net dipole is zero