Answer:
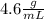
Step-by-step explanation:
The formula for finding a object's density is:
D = M/V, which means Density equals Mass divided by Volume.
In this case, our mass is 12.5 grams, our volume is 2.7mL, and our density is the unknown we are solving for.
One important thing to keep in mind when finding density is the type of measurements used. Fortunately in this case, we are dividing grams by ml, which have a 1:1 conversion rate.
To find our answer, we just divide 12.5 by 2.7 to get:
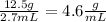
The true answer is 4.629, where 629 is repeating. However when we apply significant figures, we see our lowest amount of sig fig is 2 digits(in 2.7), so we round to 2 digits in our answer.
Hope this helps!