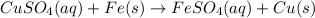Answer:
Step-by-step explanation:
Identify all of the compounds present:
- aqueous copper(II) sulfate: we have copper charged as 2+, sulfate anion has a charge of 2-. This means the charges are balanced if we take 1 ion of each to give
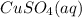 ;
; - solid iron metal: iron metal is Fe, then including the state of iron, we have
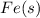 ;
; - aqueous iron(II) sulfate: iron has a charge of 2+, sulfate has a charge of 2-, so the positive charge balances the negative charge to give a formula of
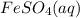 ;
; - solid copper metal: copper metal is Cu, then including the state of copper, we have
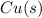 .
.
We then have a reaction: