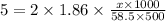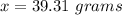Answer:
39.31 grams of NaCl
Step-by-step explanation:
Let x be the mass of NaCl added.
Decrease in freezing point = ΔTf = 5°C
Kf for water = 1.86 °C/m
Molality of the solution = m =
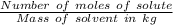
i = Vant hoff factor =
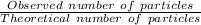
Formula for depression in freezing point:
ΔTf = i×Kf×m
i for NaCl = 2 (As NaCl dissociates into Na+ and Cl- ions in solution)
molar mass of NaCl = 58.5 g/mol