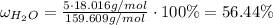Answer:
56.44 %
Step-by-step explanation:
The formula of copper(II) sulfate pentahydrate is
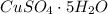 .
.
In order to calculate the mass percentage of water in it, we may assume that we have 1 mol of copper(II) sulfate pentahydrate. In general, the mass percentage is a ratio between the mass of a component and the total mass of the compound expressed in percent:
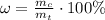
Since we're taking 1 mole of a substance here, we may state that the mass percentage of water will be calculating using molar masses instead:
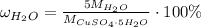
Notice that we take 5 molar masses of water, as 1 mole of copper(II) sulfate pentahydrate contains 5 moles of water molecules, then: