Answer:
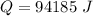
Step-by-step explanation:
Given:
- mass of water,
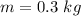
- initial temperature of water,
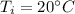
- final temperature of water,
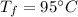
- specific heat of water,
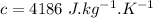
Now the amount of heat energy required:
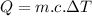
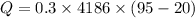
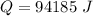
Since all of the mechanical energy is being converted into heat, therefore the same amount of mechanical energy is required.