Answer:
Ammonia does not have a trigonal shape.
Step-by-step explanation:
According to VSEPR theory:
Molecules with 3 sigma bonds and no lone pair possess trigonal planar shape irrespective of the number of pi bonds.
- Ammonia
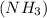 has 3 sigma bonds and one lone pair.Hence its shape is pyramidal due to bond pair - lone pair repulsion.
has 3 sigma bonds and one lone pair.Hence its shape is pyramidal due to bond pair - lone pair repulsion. - Formaldehyde (HCHO) has 3 sigma bonds and 1 pi bond.Hence it has trigonal planar shape.
- Sulfur trioxide
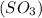 has 3 sigma bonds and 3 pi bonds.
has 3 sigma bonds and 3 pi bonds. - Boron triflouride (BF_3) has 3 sigma bonds.
Hence except ammonia all other molecules given possess trigonal planar shape.