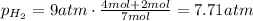Answer:
7.71 atm
Step-by-step explanation:
Given the following data:
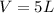

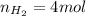
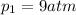
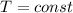
According to the ideal gas law, we know that the product between pressure and volume of a gas is equal to the product between moles, the ideal gas law constant and the absolute temperature:
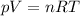
Since the temperature and the ideal gas constant are constants, as well as the fixed container volume of 5 L, we may rearrange the equation as:
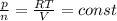
This means for two conditions, we'd obtain:
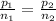
Given:
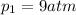
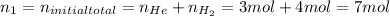
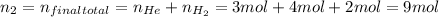
Solve for the final pressure:
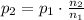
Now, according to the Dalton's law of partial pressures, the partial pressure is equal to the total pressure multiplied by the mole fraction of a component:
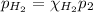
Knowing that:
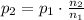
And:
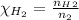
The equation becomes:
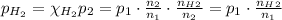
Substituting the variables: