Answer:
35.3124 g is the maximum mass of
 that can be produced.
that can be produced.
Step-by-step explanation:
The formula for the calculation of moles is shown below:

For
 :-
:-
Mass of
 = 52.3 g
= 52.3 g
Molar mass of
 = 17.031 g/mol
= 17.031 g/mol
The formula for the calculation of moles is shown below:

Thus,
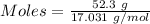
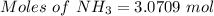
For
 :-
:-
Given mass of
 = 52.3 g
= 52.3 g
Molar mass of
 = 31.9898 g/mol
= 31.9898 g/mol
The formula for the calculation of moles is shown below:

Thus,
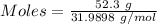
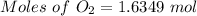
According to the given reaction:
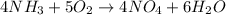
4 moles of
 reacts with 5 moles of
reacts with 5 moles of

1 mole of
 reacts with 5/4 moles of
reacts with 5/4 moles of

Also,
3.0709 moles of
 reacts with
reacts with
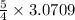 moles of
moles of

Moles of
 = 3.8386 moles
= 3.8386 moles
Available moles of
 = 1.6349 moles
= 1.6349 moles
Limiting reagent is the one which is present in small amount. Thus,
 is limiting reagent.
is limiting reagent.
The formation of the product is governed by the limiting reagent. So,
5 moles of
 on reaction forms 6 moles of
on reaction forms 6 moles of

1 mole of
 on reaction forms 6/5 moles of
on reaction forms 6/5 moles of

Thus,
1.6349 mole of
 on reaction forms
on reaction forms
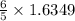 moles of
moles of

Moles of
 = 1.9618 moles
= 1.9618 moles
Molar mass of
 = 18 g/mol
= 18 g/mol
Mass of sodium sulfate = Moles × Molar mass = 1.9618 × 18 g = 35.3124 g
35.3124 g is the maximum mass of
 that can be produced.
that can be produced.