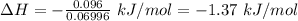Answer:
-1.37 kJ/mol
Step-by-step explanation:
The expression for the calculation of the enthalpy of dissolution of [tex[NH_4NO_3[/tex] is shown below as:-
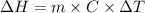
Where,
 is the enthalpy of dissolution of [tex[NH_4NO_3[/tex]
is the enthalpy of dissolution of [tex[NH_4NO_3[/tex]
m is the mass
C is the specific heat capacity
 is the temperature change
is the temperature change
Thus, given that:-
Mass of ammonium nitrate = 5.60 g
Specific heat = 4.18 J/g°C
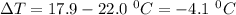
So,

Negative sign signifies loss of heat.
Also, 1 J = 0.001 kJ
So,
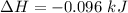
Also,
Molar mass of [tex[NH_4NO_3[/tex] = 80.043 g/mol
The formula for the calculation of moles is shown below:

Thus,
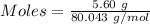
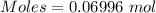
Thus,