Answer:
B) 16.4
Step-by-step explanation:
Given that:-
Current, I = 15.0 A
Time, t = 60.0 minutes
Also, 1 minute = 60 seconds
So, t =
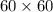 s = 3600 s
s = 3600 s
F is Faraday constant = 96485 C
Atomic weight of Nickel = 58.69 g/mol
Also, Charge on Ni in
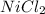 = 2
= 2
So, equivalent weight of Ni , E =
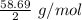 = 29.34 g/mol
= 29.34 g/mol
Thus, according to the Faraday's Law:-
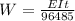
Where, W is the mass of the metal deposited.
So,
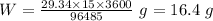
Weight of Ni metal plated out = 16.4 g