Answer:
1.
![pH = -log[H^+]](https://img.qammunity.org/2020/formulas/chemistry/college/gny7xuakxqc45obx08p22znzokdd3vdn81.png)
2. Acidic: pH < 7.00, neutral: pH = 7.00, basic: pH > 7.00
3. pH + pOH = 14.00 at room temperature
Step-by-step explanation:
Firstly, it is true that pH is defined as the negative logarithm of the concentration of hydronium ions. A simple rule for any p-function in chemistry is to remember that p = -log. For example:
![pH = -log[H^+], pK_a = -log(K_a)](https://img.qammunity.org/2020/formulas/chemistry/college/fa8lbf9l7g8zwqrd16r9739o9cipsqpvhs.png) etc.
etc.
Secondly, neutral solutions are the ones which have equal concentrations of hydronium and hydroxide ions regardless of the temperature. At room temperature, specifically, for pure solutions:
![[H_3O^+] = [OH^-] = 1.00\cdot 10^(-7) M](https://img.qammunity.org/2020/formulas/chemistry/college/pq5iw3b8pk176a3py8co0u8a5hfw8iucz0.png)
Notice that applying the equation above:
![pH = -log[H_3O^+] = -log(1.00\cdot 10^(-7)) = 7.00](https://img.qammunity.org/2020/formulas/chemistry/college/avixk5wgjso5ojd0fa6wlp8j9fagrwo1wd.png)
This means neutral solutions at room temperature have a pH value of 7.00.
Now, let's say the concentration of hydronium increases to a value of:
![[H_3O^+] =2.00\cdot 10^(-7) M](https://img.qammunity.org/2020/formulas/chemistry/college/6zo6dt0cydcdtt5ddz33606jtcfk7cmqe9.png)
Then:
![pH = -log[H_3O^+] = -log(2.00\cdot 10^(-7)) = 6.70](https://img.qammunity.org/2020/formulas/chemistry/college/nwusuylce8tnmfzikjj40ulnskm4qk7zwk.png)
Notice that an increase in the molarity of hydronium lead to a decrease in pH. Therefore, acidic solutions have a pH < 7.00, while basic solutions have a pH > 7.00. Neutral solution was described as the one having pH = 7.00.
Thirdly, ion-product constant of water is defined as:
![K_w=[H_3O^+][OH^-]](https://img.qammunity.org/2020/formulas/chemistry/college/al6742x2k8ppf6dw9go0ejnbsp2dxs31wf.png)
The given value of:
![K_w=[H_3O^+][OH^-]=1.00\cdot10^(-14)](https://img.qammunity.org/2020/formulas/chemistry/college/4xzcx4xdqgmv4l0uk7wxnc83xlki8q1joi.png)
Is only valid at room conditions. Now let's take the -log of both sides:
![-log(K_w)=-log([H_3O^+][OH^-])](https://img.qammunity.org/2020/formulas/chemistry/college/os8rkknryvnnsq9dsk0a2kfif8ov95ynlr.png)
Apply the rule of logs:
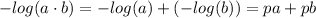
We obtain:
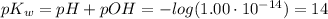
That said, the equation is proved.