Answer:
-13094.55179 J
Step-by-step explanation:
Q = Heat =
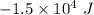
P = Pressure =
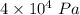
 = Change in volume =
= Change in volume =
 (negative because it is decreasing)
(negative because it is decreasing)
h = Height = 16.3 cm
r = Radius = 30.5 cm
Entropy is given by
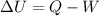
Work done is given by
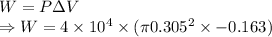
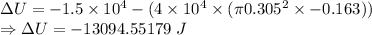
The change in the internal energy of the system is -13094.55179 J