The question is incomplete, here is a complete question.
Calculate the standard entropy of vaporization of ethanol at its boiling point 352 K. The standard molar enthalpy of vaporization of ethanol at its boiling point is 40.5 kJ/mol.
Answer : The standard entropy of vaporization of ethanol is, 115 J/mol.K
Explanation :
Formula used :
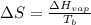
where,
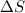 = change in entropy
= change in entropy
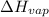 = change in enthalpy of vaporization = 40.5 kJ/mol
= change in enthalpy of vaporization = 40.5 kJ/mol
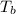 = boiling point temperature = 352 K
= boiling point temperature = 352 K
Now put all the given values in the above formula, we get:
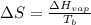

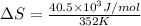
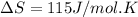
Therefore, the standard entropy of vaporization of ethanol is, 115 J/mol.K