Answer: In the above reaction,
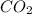 gas is formed in the reaction.
gas is formed in the reaction.
Step-by-step explanation:
We are given:
Nitric acid
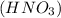 is dropped in calcium carbonate
is dropped in calcium carbonate
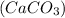 , a gas is released.
, a gas is released.
The chemical equation for the reaction of nitric acid and calcium carbonate follows:
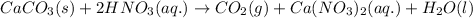
By Stoichiometry of the reaction:
1 mole of calcium carbonate reacts with 2 moles of nitric acid to produce 1 mole of carbon dioxide, 1 mole of calcium nitrate and 1 mole of water molecule.
Carbon dioxide gets released in the form of effervescence (bubbles).
Hence, in the above reaction,
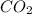 gas is formed in the reaction.
gas is formed in the reaction.