Answer:
Rise in temperature will be
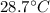
So option (c) will be the correct answer
Step-by-step explanation:
We have given solar radiation on the panels
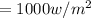
25 % of the conversion efficiency = 25 % 1000 = 250
Area is given
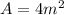
So radiated power
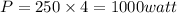
Time is given t = 1 hour = 3600 sec
So energy
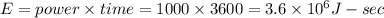
Mass of water m = 30 kg
And
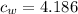
So rise in temperature
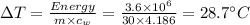
So option (c) will be the correct option