Step-by-step explanation:
1.
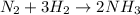
According to reaction , 1 molecule of nitrogen gas reacts with 3 molecules of hydrogen gas to give 2 molecules of ammonia
Then 4 molecules of nitrogen gas will react with:
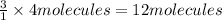 of hydrogen gas.
of hydrogen gas.
As we can see that molecules of nitrogen gas are in excess amount.
So, the molecules of ammonia formed will depend upon molecules of hydrogen gas.
According to reaction, 3 molecules of hydrogen gas gives 2 molecules of ammonia.
Then 4 molecules of hydrogen gas will give :
=
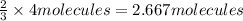 of ammonia
of ammonia
2)
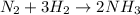
Molecules of nitrogen gas in balanced chemical equation = 1
Molecules of hydrogen gas in balanced chemical equation = 3
The ratio of nitrogen and hydrogen molecules would result in no left-over reactants will be:
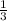 = 1 : 3
= 1 : 3
For every 1 molecule of nitrogen gas molecules 3 molecules of hydrogen gas molecules are required to form.
3)
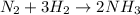
Moles of nitrogen gas =
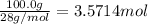
Moles of hydrogen gas =
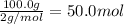
According to reaction , 1 mole of nitrogen gas reacts with 3 mole of hydrogen gas to give 2 moles of ammonia
Then 3.5714 moles of nitrogen gas will react with:
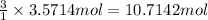 of hydrogen gas.
of hydrogen gas.
As we can see that moles of nitrogen gas are only reacting with 10.7142 moles of hydrogen gas which means that nitrogen gas is limiting reagent and hydrogen gas is excessive reagent.
So, amount of ammonia gas will depend upon the moles of nitrogen gas.
According to reaction, 1 molecules of nitrogen gas gives 2 molecules of ammonia.
Then 3.5714 mol of nitrogen gas will give :
=
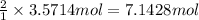 of ammonia
of ammonia
Theoretical yield = Mass of 7.1428 moles of ammonia:
7.1428 mol × 17 g/mol = 199.9 g
199.9 g is the theoretical yield of the reaction.
Hydrogen gas is the excess reactant.
Nitrogen gas is the limiting reactant.