Answer:
35.42 moles of magnesium are used.
Step-by-step explanation:
Initial amount of moles ion container = 50 moles
Final moles of magnesium left in the container : n
Mass of the magnesium after partial use = 350 g
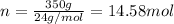
Moles of magnesium used :
= Initial moles of magnesium left - final moles of magnesium
= 50 moles - 14.58 moles = 35.42 moles
35.42 moles of magnesium are used.