Step-by-step explanation:
It is given that the given system contains
 ,
,
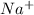 , and
, and
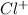 .
.
Therefore, formula for number of components is given as follows.
C = n - E
where, E = no. of independent equation relating the concentration of species
n = number of species involved
Now, put the given values into the above formula as follows.
C = n - E
= 3 - 1
= 2
Since, NaCl when dissolves in water leads to the formation of a homogeneous mixture. Hence, then phase will be equal to 1.
thus, we can conclude that in the given system there are 2 components present in 1 phase.