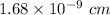Answer:
The atomic radius is:-
Step-by-step explanation:
The expression for density is:
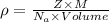

M is molar mass of polonium = 209 g/mol
For Simple Cubic unit cell , Z= 1
 is the density = 9.142 g/cm³
is the density = 9.142 g/cm³
Thus,
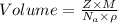
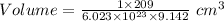
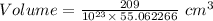
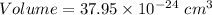
Also, Volume =
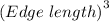
Thus, edge length =
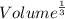 =
=
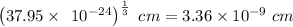
Also, for Simple Cubic unit cell,
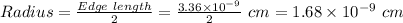
Hence, the atomic radius is:-