Answer:
2 moles of solute.
Step-by-step explanation:
Molarity is defined as number of moles of solute present in one litre of solution.
Hence one molar solution of NaCl means 1 mole of NaCl is present in 1 litre of solution.
Molarity =
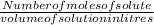
For 1 litre of solution 1 mole of solute is required to make a one molar solution.
Hence for 2 litres of solution we require 2 moles of solute to keep it one molar.