Answer:
(b) The C3H8 and CH4 molecules have the same average kinetic energy
Step-by-step explanation:
The expression for the root mean square speed is:
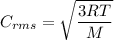
R is Gas constant having value = 8.314 J / K mol
M is the molar mass of gas
Molar mass of
 = 0.04401 kg/mol
= 0.04401 kg/mol
Thus, it depends on the molecular mass which is not the same for both species. Hence, a is not the answer.
The expression for the kinetic energy is:-
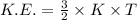
k is Boltzmann's constant =
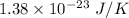
T is the temperature
Thus, kinetic energy is only proportional to the temperature. Hence, there is same value of kinetic energy for both the gases and hence, b is the answer.
Since, the velocity are different for both gases, the rate cannot be same. Hence, c is not the answer.
Considering ideal gas equation as:

where,
P is the pressure
V is the volume
n is the number of moles
T is the temperature
R is Gas constant having value = 0.0821 L atm/ K mol
Thus, pressure is directly proportional to the number of moles and hence, they don't not have same pressure. Thus, d is not the answer.