Answer: The given statement is true.
Step-by-step explanation:
For the reaction to be spontaneous, the Gibbs free energy of the reaction must come out to be negative.
The equation used to calculate Gibbs free energy follows:
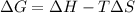
Exothermic reactions are defined as the reactions in which energy is released in the form of heat. The enthalpy change
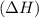 of the reaction comes out to be negative for this kind of reaction.
of the reaction comes out to be negative for this kind of reaction.
Entropy change is defined as the change in the measure of randomness in the reaction. It is represented as
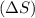 . Randomness of gaseous particles is more than that of liquid which is further more than that of solids.
. Randomness of gaseous particles is more than that of liquid which is further more than that of solids.
We are given:
A solid substance is converting into a gaseous substance.

As, the entropy is increasing. So, the entropy change is positive.
Thus, the above reaction is spontaneous.
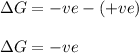
Hence, the given statement is true.