Answer : The mass percent of
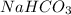 in the sample is, 65.7 %
in the sample is, 65.7 %
Explanation :
As we are given that Na makes up 18.00% of the entire mass of the sample.
First we have to calculate the mass percent of Na in
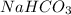 .
.
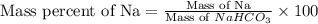
Mass of Na = 23 g
Mass of
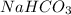 = 84 g
= 84 g
Putting values in above equation, we get:
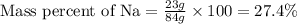
Now we have to calculate the mass percent of
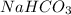 in the sample.
in the sample.
As, 27.4 % amount of Na present in 100 %
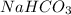
So, 18 % amount of Na present in
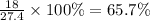
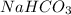
Therefore, the mass percent of
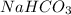 in the sample is, 65.7 %
in the sample is, 65.7 %