Answer:
2.5 M NaOH
Step-by-step explanation:
The solution with the highest molarity is the most concentrated one.
Molarity is the number of moles per unit volume of a solution.
- Molarity =
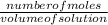
2.5M implies there are 2 mole of NaOH per solution of this given substance.
This is the highest from the list as other ones falls below it.
The lowest concentration is 0.2M because just 0.2 mole of NaOH is contained per unit volume.