"Argon is a gas that may leak from a sample and provide inaccurate data" is problematic about the potassium-argon method of radiometric dating.
Answer: Option D
Explanation:
Similar to carbon dating, now a day’s researchers or more specifically archaeologist are keen to use potassium-argon method of radiometric dating. This is because the half life time of potassium-40 is
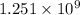 which is large compared to the half-life of carbon i.e 5730 years. So, there is high chance of detecting potassium with argon mixture in monuments or objects dated more than 5730 years.
which is large compared to the half-life of carbon i.e 5730 years. So, there is high chance of detecting potassium with argon mixture in monuments or objects dated more than 5730 years.
Also the accuracy will be high. But the disadvantage is the argon which being a gas has the tendency of leakage. Also, argon is a noble gas so there is less chance of reaction so sometimes this leakage and non-reactivity of argon can lead to inaccuracy in data.