Answer:
Only one atom are bonded to the central atom in the
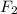 .
.
Step-by-step explanation:
Fluorine most electronegative element.
The atomic number of fluorine is 9.
Electronic configuration -
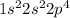
Hence, it required only one electron to get noble gas[Ne] electronic configuration.
In the
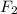 molecule two fluorine atoms are bonded by single covalent bond to get stability.
molecule two fluorine atoms are bonded by single covalent bond to get stability.
Each atom share one electron and form a bond.
The structure of
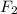 molecule is as follows.
molecule is as follows.