The question is incomplete, here is a complete question.
A sample of impure tin of mass 0.538 g is dissolved in strong acid to give a solution of
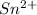 . The solution is then titrated with a 0.0448 M solution of
. The solution is then titrated with a 0.0448 M solution of
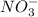 , which is reduced to NO(g). The equivalence point is reached upon the addition of
, which is reduced to NO(g). The equivalence point is reached upon the addition of
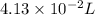 of the
of the
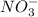 solution.
solution.
Find the percent by mass of tin in the original sample, assuming that it contains no other reducing agents.
Answer : The percent mass of tin in the original sample is 61.3 %
Solution :
First we have to calculate the moles of
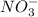 .
.
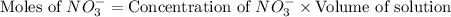
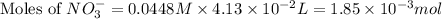
Now we have to calculate the moles of
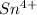
The balanced chemical reaction is,
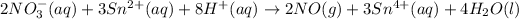
From the reaction, we conclude that
As, 2 mole of
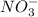 react with 3 mole of
react with 3 mole of
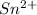
So,
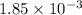 moles of
moles of
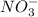 react with
react with
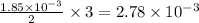 moles of
moles of
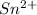
Now we have to calculate the mass of
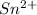
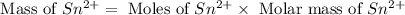
Molar mass of tin = 118.71 g/mol
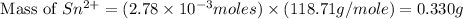
Mass of
 reacted = 0.330 g
reacted = 0.330 g
Original mass of
 = 0.538 g
= 0.538 g
Now we have to calculate the percent mass of tin in the original sample.
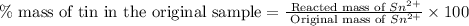
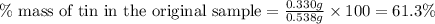
Therefore, the percent mass of tin in the original sample is 61.3 %