Answer: The heat required for the given process is 2659.3 kJ
Step-by-step explanation:
The processes involved in the given problem are:
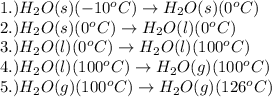
Pressure is taken as constant.
To calculate the amount of heat absorbed at different temperature, we use the equation:
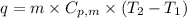 .......(1)
.......(1)
where,
q = amount of heat absorbed = ?
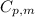 = specific heat capacity of medium
= specific heat capacity of medium
m = mass of water/ice
 = final temperature
= final temperature
 = initial temperature
= initial temperature
To calculate the amount of heat released at same temperature, we use the equation:
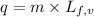 ......(2)
......(2)
where,
q = amount of heat absorbed = ?
m = mass of water/ice
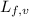 = latent heat of fusion or vaporization
= latent heat of fusion or vaporization
Calculating the heat absorbed for each process:
We are given:
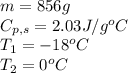
Putting values in equation 1, we get:

Converting the latent heat of fusion in J/g, we use the conversion factor:
Molar mass of water = 18 g/mol
1 kJ = 1000 J
So,
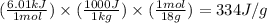
We are given:
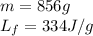
Putting values in equation 2, we get:
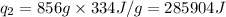
We are given:
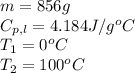
Putting values in equation 1, we get:
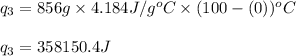
Converting the latent heat of vaporization in J/g, we use the conversion factor:
Molar mass of water = 18 g/mol
1 kJ = 1000 J
So,
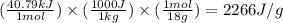
We are given:
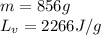
Putting values in equation 2, we get:

We are given:
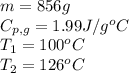
Putting values in equation 1, we get:
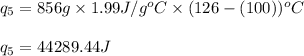
Total heat absorbed =
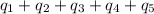
Total heat absorbed =
![[31278.24+285904+358150.4+1939696+44289.44]J=2659318.08J=2659.3kJ](https://img.qammunity.org/2020/formulas/chemistry/high-school/r5ad9yuhgvjhsez186gp0sv0cia2yk9f2l.png)
Hence, the heat required for the given process is 2659.3 kJ