Answer:
390.34 g of glucose will be produced if 13 moles of carbon dioxide react.
Step-by-step explanation:
Moles of carbon dioxide = 13 moles
According to the reaction shown below:-
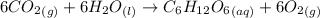
6 moles of carbon dioxide are required to produce 1 mole of glucose
Also,
1 mole of carbon dioxide is required to produce
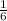 mole of glucose.
mole of glucose.
So,
13 moles of carbon dioxide are required to produce
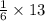 mole of glucose.
mole of glucose.
Moles of glucose produced = 2.1667 moles
Molar mass of glucose = 180.156 g/mol
The formula for the calculation of moles is shown below:

Thus,
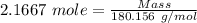
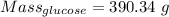
390.34 g of glucose will be produced if 13 moles of carbon dioxide react.