Answer:
The value of Standard cell potential doesnot change with the change in temperature and is a constant.
Step-by-step explanation:
According to Nernst's equation:
![E=E_0-(RT)/(nF)log([reducing.agent])/([oxidizing.agent])](https://img.qammunity.org/2020/formulas/chemistry/high-school/gmzql474nlm0vpwnzim3k82ind4emifg75.png)
where ,
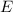 is the cell potential
is the cell potential
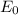 is the standard cell potential
is the standard cell potential
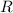 is the universal gas constant =
is the universal gas constant =

 is the number of moles
is the number of moles
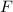 is the value of 1 faraday
is the value of 1 faraday
 is the temperature.
is the temperature.
As we see here ,
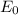 is the standard cell potential and is only calculated at a particular temperature which is
is the standard cell potential and is only calculated at a particular temperature which is
 .
.
Thus the value of
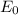 doesnot change with the change in temperature and is a constant.
doesnot change with the change in temperature and is a constant.