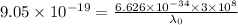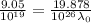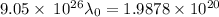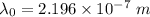Answer:
A.
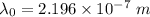
Step-by-step explanation:
The work function of the Platinum =
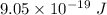
For maximum wavelength, the light must have energy equal to the work function. So,
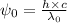
Where,
h is Plank's constant having value
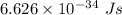
c is the speed of light having value
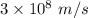
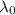 is the wavelength of the light being bombarded
is the wavelength of the light being bombarded
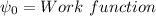
Thus,