Answer:
The volume that this same amount of air will occupy in his lungs when he reaches a depth of 124 m is - 0.27 L.
Step-by-step explanation:
Using Boyle's law
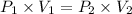
Given ,
V₁ = 3.6 L
V₂ = ?
P₁ = 1.0 atm
P₂ = 13.3 atm (From correct source)
Using above equation as:
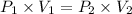
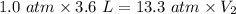
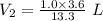
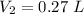
The volume that this same amount of air will occupy in his lungs when he reaches a depth of 124 m is - 0.27 L.