Answer: Reducing agent in the given reaction is
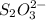 .
.
Step-by-step explanation:
A reducing agent is defined as an element which tends to lose electrons to other element leading to an increase in its oxidation number.
In the given reaction, oxidation state of sulfur in
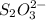 is +2 and
is +2 and
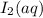 has 0 oxidation state.
has 0 oxidation state.
In
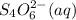 oxidation state of S is 2.5 and in
oxidation state of S is 2.5 and in
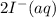 oxidation state of I is -1.
oxidation state of I is -1.
Since, an increase in oxidation state of S is occurring from +2 to +2.5. Hence, it is acting as a reducing agent.
Thus, we can conclude that reducing agent in the given reaction is
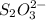 .
.