Given :
560 gram of silicon dioxide.
To Find :
Number of moles of silicon dioxide in 560 gram.
Solution :
We know, molecular mass silicon dioxide is 60 gram/mol.
So, number of moles of silicon dioxide in 560 gram is :
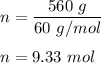
Now, one mole of any compound contain
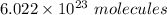 .
.
So, number of molecules in 9.33 moles of silicon dioxide is :
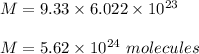
Hence, this is the required solution.