Answer:
3.73994
No,
Step-by-step explanation:
 = Pressure at the bottom of the lake = 3.5 atm
= Pressure at the bottom of the lake = 3.5 atm
 = Pressure at the top of the lake = 1 atm
= Pressure at the top of the lake = 1 atm
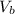 = Volume at the bottom of the lake
= Volume at the bottom of the lake
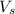 = Volume at the top of the lake
= Volume at the top of the lake
 = Temperature at the bottom of the lake = 4 °C
= Temperature at the bottom of the lake = 4 °C
 = Temperature at the top of the lake = 23 °C
= Temperature at the top of the lake = 23 °C
From ideal gas law we have the relation
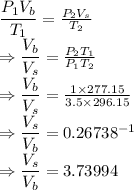
The ratio is 3.73994
As Jacques is ascending if he holds his breath his lungs acting like a bubble would expand. Hence, it is not safe to hold his breath while ascending,