Answer : The nutritional Calories present per gram of the candy are 5.48 cal/g.
Explanation :
First we have to calculate the heat given off by the combustion of this candy.
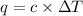
where,
q = heat released by the reaction = ?
c = specific heat of calorimeter =
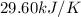
 = change in temperature =
= change in temperature =
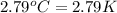 (Change in kelvin temperature = Change in Celsius temperature)
(Change in kelvin temperature = Change in Celsius temperature)
Now put all the given values in the above formula, we get:
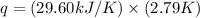
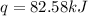
Now we have to convert heat from kJ to cal.
Conversion used : 1 cal = 4.186 kJ
As, 4.186 kJ heat = 1 cal
So, 84.58 kJ heat =
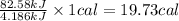
Now we have to calculate the nutritional Calories are there per gram of the candy.
Amount of nutritional Calories =

Therefore, the nutritional Calories present per gram of the candy are 5.48 cal/g.