Answer: The standard enthalpy change for the reaction is -16.26 kJ
Step-by-step explanation:
Enthalpy change is defined as the difference in enthalpies of all the product and the reactants each multiplied with their respective number of moles. The equation used to calculate enthalpy change is of a reaction is:
![\Delta H^o_(rxn)=\sum [n* \Delta H^o_f_((product))]-\sum [n* \Delta H^o_f_((reactant))]](https://img.qammunity.org/2020/formulas/chemistry/high-school/5c7vs79qepq19tdktywtbtker28hpuplvy.png)
For the given chemical reaction:
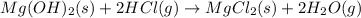
The equation for the enthalpy change of the above reaction is:
![\Delta H^o_(rxn)=[(1* \Delta H^o_f_((MgCl_2(s))))+(2* \Delta H^o_f_((H_2O(g))))]-[(1* \Delta H^o_f_((Mg(OH)_2(s))))+(2* \Delta H^o_f_((HCl(g))))]](https://img.qammunity.org/2020/formulas/chemistry/college/nls1apjrng6nv2vpft3edkyhpk44bxhd0y.png)
We are given:
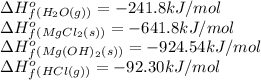
Putting values in above equation, we get:
![\Delta H^o_(rxn)=[(1* (-641.8))+(2* (-241.8))]-[(1* (-924.54))+(2* (-92.30))]\\\\\Delta H^o_(rxn)=-16.26kJ](https://img.qammunity.org/2020/formulas/chemistry/college/76p3subnwzgaafdluyhyatrwlkc68aeroj.png)
Hence, the standard enthalpy change for the reaction is -16.26 kJ