Answer:
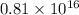 beta particles
beta particles
Step-by-step explanation:

Given mass = 14.0 g
Molar mass = 137 g/mol
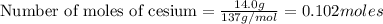
According to avogadro's law, 1 mole of every substance weighs equal to its molecular mass and contains avogadro's number
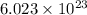 of particles.
of particles.
1 mole of cesium contains atoms =
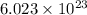
0.102 moles of cesium contains atoms =
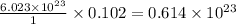
The relation of atoms with time for radioactivbe decay is:
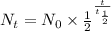
Where
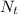 =atoms left undecayed
=atoms left undecayed
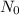 = initial atoms
= initial atoms
t = time taken for decay = 3 minutes
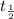 = half life = 30.0 years =
= half life = 30.0 years =
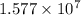 minutes
minutes
The fraction that decays :
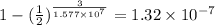
Amount of particles that decay is =

Thus
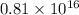 beta particles are emitted by a 14.0-g sample of cesium-137 in three minutes.
beta particles are emitted by a 14.0-g sample of cesium-137 in three minutes.